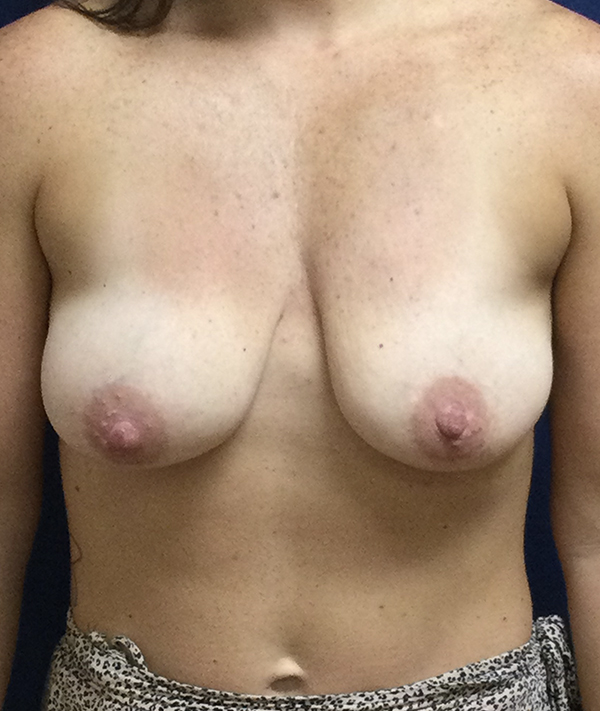
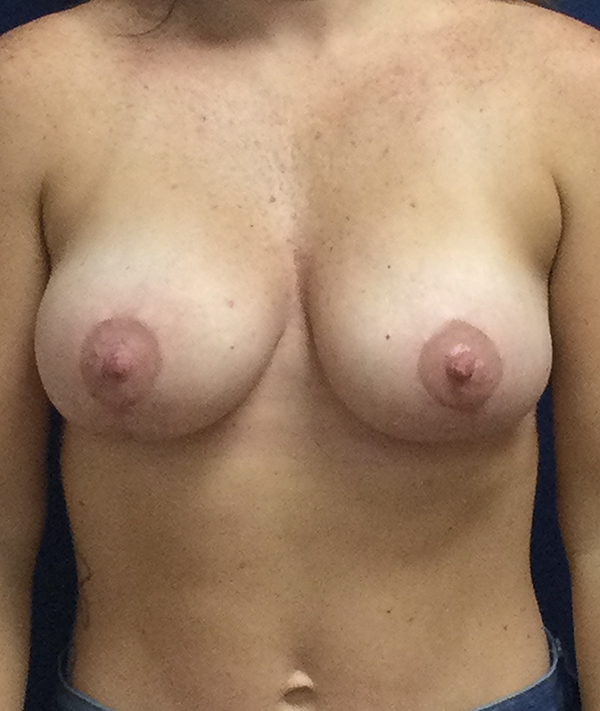
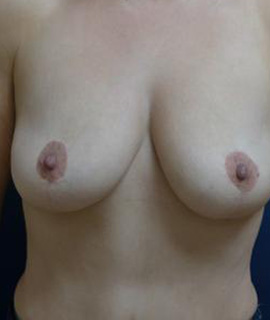
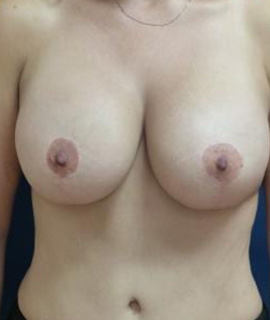
Achieve the curves you've always wanted with personalized breast augmentation in Houston, TX. Whether you’re seeking fuller volume, improved symmetry, or a restored shape, our board-certified surgeons offer advanced techniques and natural-looking results. We provide a wide selection of breast implants in Houston, TX to match your body and aesthetic goals, helping you feel confident and beautiful in your own skin.
What are the benefits of breast augmentation?
- Wide variety of implant shapes and sizes to choose from
- Helps correct changes in the upper body due to aging and pregnancy
- Improves wardrobe options
- Creates a more youthful, attractive physique
- Enhances self-confidence
- Can help combat volume loss in the breasts
- Long-lasting results
- Can be well combined with mastopexy